CO2 emissions are ruining C14 readings

Radiocarbon dating method is losing its validity
CO2-emissions from automobiles, factories and power-stations are robbing scientists of an important instrument which they use to determine the age of objects. The important Radiocarbon dating method is losing its validity, according to Heather Graven of Imperial College in London.
The method establishes the amount of C14, a radioactive variant of carbon, in wood, bones or fabric. C14 decays at a constant rate. Its quantity in relation to the stable, much more frequent carbon-isotope C12 shows therefore how old an object is.
C14 forms in the higher layers of the atmosphere under the action of cosmic radiation. Living beings absorb C14 together with CO2. As soon as they die, the supply of C14 stops – and the radiocarbon-clock starts ticking. The C14 contained in the plant or in the bone under consideration starts decaying with a half-live value of 6,000 years – at which time therefore only one half of the C14-particles will still be present.
The artificial supply of CO2 from exhaust-emissions changes the relation between C12 and C14 in the atmosphere: the emissions stem from coal, gas or oil, which was formed millions of years ago, they do not contain any C14, as the isotope which would have been originally present would have completely decayed after a few tens of thousand years.
So that substances which are presently absorbing CO2 from the air contain little C14 to begin with, but an unnaturally high amount of C12. The consequence: they are practically artificially aged – the radiocarbon dating method shows them to be very old.
If measured by the radiocarbon-method, the air is „aging“ every year by an amount of 30 years, Heather Graven writes in the scientific magazine "Proceedings of the National Academy of Sciences." This artificial aging leads to old objects no longer being easily distinguishable from new objects, and ever and ever less so.
The radiocarbon age of organic materials which will be produced in 2050, will resemble the one of objects one thousand years old. By 2100, organic materials will show from the beginning an age of 2000 years – the radiocarbon dating method will be incapable of distinguishing whether a wooden object originated in Ancient Rome or if it was recently made.
Axel Bojanowski, Der Spiegel, July 21, 2015
Translated from the German by Anne-Marie de Grazia
Go to original article in German
Alfred de Grazia: Collapsing tests of time - C14 dating (from: Chaos & Creation)
Cosmic rays of the galaxy strike and explode atoms of the atmosphere. These give off neutrons that interact with nitrogen of the air to make carbon-14 or C14. This passes into carbon dioxide and then into plants and other living organisms through their food supply. Living organisms also ingest carbon-12 which does not decay. When anything that has lived dies, it ceases to ingest radioactive carbon-14, and the carbon-14 within its cells begins to decay into nitrogen-14. Half of it might decay in 5,730 years, the other half in another 85,000 years, according to conventional theory.
Thus, any once-living organic substance can be tested for the amount of C14 that it now contains in relation to the amount that was originally ingested. The carbon-12 level can be used as the base of measurement. However, not all species ingest 14C in the same amounts, so that specific rates must be calculated for different species. More importantly, "the amount that was originally ingested" may vary [1].
All that has been said about the effects of high-energy forces upon the atmosphere applies to carbon-14. How much nitrogen was in the primeval atmosphere is unknown and is presumed on today's measure. The carbonization (burning) of the biosphere and the sudden proliferation of flora will directly affect the rate of generation of C14. Also, if C14 was heavily generated in the atmosphere by electrical phenomena and radio storms, in the times when Uranus, Saturn and Jupiter were worshipped, it was ingested extensively by organisms. Matter of this period would test as "younger" today, provided that several other "constants" remained constant.
As disasters diminish in intensity following chaos, relatively less C14 would be created; matter would grow "older." Several disasters involved the desiccation or saline ruination of large areas of the world; this would cause less carbon dioxide to discharge from plants. During short periods of burning, great amounts of non-radioactive carbon are discharged into the air and waters, and therefore contribute to a temporary "aging" of the new life of the time that follows. Whenever both a cosmic brilliancy and a conflagration occurred, today's tests would be contradictory, and averages would mislead.
Libby and Lukens have estimated a "perturbation of about 1%" occurring in the production of radiocarbon of tree rings by lightning bolts[2]. This represents a neutron supply added to the supply produced by cosmic rays. The estimates are based upon present-day assumptions, also upon highly varied conditions and inexact knowledge of the extent of lightning or its effects[3] . It does not consider lightning discharges occurring solely in the atmosphere, and especially the mega-bolts that can be a thousand times more powerful than the average earth-striking bolt and were recently discovered by satellites.
Aside from what is happening in the biosphere, a fixed C14 component of the atmosphere, upon which the test is based, depends upon a constant encounter rate between cosmic particles and nitrogen that produces C14. Since radiation storms occurred and long-term radiation levels were diminished and increased from time to time, intervals of the C14 scale must have been rendered invalid, except for mere coincidence. Only in the years from about 500 B. C. TO A. D. 1900 might the amount taken in by organisms have acquired some constancy. Even so, strange aberrations of the C14/C12 ratio occur, as with shellfish and coral growths.
Figure 5.
RADIOCARBON DATINGS AS INDICATORS OF ECOLOGICAL STRESS.
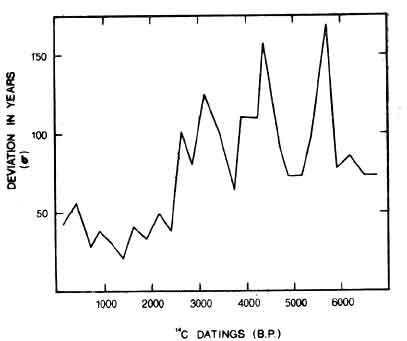
The left-hand scale (s) registers the standard deviation of the "true" curve from the trend curve - the number of years by which the radio carbon dates of each 250 year period deviate from the average of the whole group of dates of that period. The bottom scale represents the years before the present (taken as 1950 A. D.). As the chart shows, the dates begin to be erratic increasingly around the time of the Martian encounters (-2687 B. P. by this book's 2000 B. P. standard). The time scale goes to -6750, and thus carries one through the Martian, Venusian, Mercurian, and Jovean ages. However, even the erratic swings shown here do not portray the true extent of atmosphere and ecological disturbance, because, as the text asserts, a succession of quick changes in the atmosphere is possible, from low to high radiocarbon intake therefore by the biosphere, and this phenomenon would cause an evening-out of still a second and possibly much more serious form of deviation. Within a time of several years, an organism could ingest widely varying amounts of C14. Hence I suggest that radiocarbon dating may be useless before about 2500 years ago and there may have been a completely different radiocarbon cycle, as Melvin Cook maintains, before the Lunarian catastrophes.
(Source: Damon et al, extended and applied [47] by G. W. Oosterhout Half-life is 5730 years.)
There are many anomalies in C14 dating, a few of which are mentioned elsewhere in [Chaos and Creation.] Artifact dating has become quite common, with most of the apparent successes occurring on artifacts and substances of the recent historical past. But it is precisely the problem of C14 dating that, by our theory, it is almost surely wrong in the earlier periods when the tests are most needed.
A group of scientists recently excavated "Little Salt Spring, Florida: A Unique Underwater Site." Among many remains they found in a lower level a tortoise carapace, which provided a date of 13,450 ± 190 B. P., and a wood stake used to pry open the animal, which gave a date of 12,030 ± 200. Some 1400 years of difference. Yet this is not the only problem. The whole range of time may be in question. For a base of a carved oak mortar was discovered and dated to 9080 B. P. and then declared to be similar in style to a piece recovered at Key Marco, 130 km to the South, and dated at about 1200 years ago [4] .
The quantavolutionary hypothesis is disruptive of carbondating, as it has been conceived. An adjusted curve is impossible because the revolutions of the atmosphere in precisely the most critical millenia in primevalogy cannot be positioned and defined sufficiently well for them to be employed in weighing the scale intervals. The C14 method will be useful for dating the past 2,400 years, when allowances are made for short-term atmospheric fluxes owing to extraordinary cosmic, volcanic, solar, industrial, nuclear explosional, or other activity disturbing to the atmosphere.
Mysteriously, corroboration of some of our conclusions comes from a retrogressive calculation by Melvin Cook of the amount of C14 in the ancient atmosphere. Granted the present level of carbon-14 and the fact that it is rising slightly, he found that all the 14C would have had to arrive in the atmosphere within the past ten to twelve thousand years. [5] Far from being constant, prior radio-carbon was at this point wiped out statistically and theoretically a new atmospheric accumulation began. This would appear to be about the time of the climactic Lunarian catastrophe. However, this calculation, as Dr. Cook might grant is more useful as a reductio ad absurdum than as a plotting of the true history of atmospheric carbon.
In: Chaos and Creation (1979).
Notes
1) Damon (1972); Oosterhout (1976).
2) (1973), 5903.
3) Komarek (1964), (1971); "Lightning Superbolts...." (1977).
4) Clausen et al. (1979), 611.
5) Cook (1970).

