Neanderthal environmental bio-advantages and their socio-cultural effects
Environmental stress factors and Neanderthal DNA (2/3)
by Amanda Laoupi
The disappearance of the Neanderthals is one of the most intriguing, controversial and complicated issues in Palaeolithic Archaeology, as well as in Palaeoanthropology worldwide, having given birth to a plethora of hypotheses, ranging from the inability of Neanderthals to cope with climate change, competitive exclusion or even genocide by anatomically modern humans, to hybridization with the Cro-Magnon populations (Laoupi, 2016). Generally speaking, Neanderthals (Homo Neanderthalensis) coexisted with anatomically modern humans for several thousand years before their ‘disappearance.’ Biological, anatomical, pathological, climatic, environmental and social factors seem to have interdependently contributed to this phenomenon. Nevertheless, the topic per se is beyond the scope of this paper.
In reality, even though Neanderthals are extinct, they have not disappeared from the face of Earth, as their genetic imprint lives within modern humans through interbreeding (Hybridization Hypothesis). The point of divergence between Neanderthals and modern humans is detected ca at 706 Ka, as a difference in 27 nucleotides; the first proto-Neanderthal traits in Europe have appeared around 500-350 Ka, the complete ones ca 130 Ka; they seemed to disappear from Asia around 50 Ka and from Europe ca 25 Ka. The most recent archaeological evidence dates back to ca 28 Ka and it is found at Vindija Cave - Croatia (1998), while the latest skeletal remains with Neanderthal traits come from Lagar Velho in Southern Iberia, dated back to 24.5 Ka, and from the cave of Pestera Muierii, Romania (Frayer, 1992; Duarte, et al., 1999; Soficaru, et al., 2006; Trinkhaus, 2007; Jones, 2007; Stringer, 2012).
Recent genetic studies on the Neanderthal genome, composed of over 3 billion nucleotides from three individuals, conducted by an international team of researchers, indicate some form of hybridization between archaic humans and modern humans that had taken place after modern humans emerged from Africa. The Neanderthals contributed up to 4% of modern Eurasian genomes, while the Denisovans (Reed, et al., 2004; Krause, et al., 2010; Reich, et al., 2010) contributed roughly 4-6% of modern Melanesian genomes. The interbreeding must have occurred early in the migration of modern humans out of Africa, perhaps in the Middle East, around 65 to 90 Ka (Dalen, et al., 2012). No evidence for gene flow in the direction from modern humans to Neanderthals was found. These results, though, do not rule out an alternative scenario, according to which the source population of non-African modern humans was already more closely related to Neanderthals than other Africans were, due to ancient genetic divisions within Africa (Green, et al., 2010; Noonan, 2010). In addition, researchers at Oxford University and Plymouth University found evidence of Neanderthal and Denisovan viruses in modern human DNA; probably those viruses originated in our common ancestors more than 0.5 Ma (Agoni, et al., 2012; Marchi, et al., 2013).
More specifically, research has revealed many intriguing facts:
▪ Hemochromatosis (HFE) has the highest prevalence in Caucasian groups. Neanderthals would have had great advantage in the efficient absorption of iron, due to their frequent injuries during close-combat hunting, as the cure of hemochromatosis is to loose blood (Tomatsu, et al., 2003; Hollerer et al., 2017). Furthermore, hemochromatosis is now associated with the HLA (human leukocyte antigen) gene locus and identified as an autosomal recessive disease that mainly affects men (Simon et al., 1975).
▪ Comparing the HLA (human leukocyte antigen) genes of modern human populations with those from Denisovans and Neanderthals, scientists identified a handful that could be traced back to ancient sexual encounters between the groups. One variant of HLA gene, most common in West Asian populations, the region where the mating probably occured, known as HLA-B*73, likely arose in modern humans after cross-breeding with Denisovans. The Neanderthals contributed also a string of HLA gene variants, or alleles, to the modern Eurasian population's gene pool. But this intake also lead to auto-immune diseases among modern humans, e.g. a gene variant called HLA-B51, which came from cross-breeding with Neanderthals and has already been linked to Behcet's disease, a rare and chronic inflammatory condition, known also as Silk Road Disease, due to its prevalence in the areas surrounding the old silk trading routes in the Middle East and in central Asia (Simoons, 1981).
▪ Psoriasis has 1 - 2 % prevalence in European descent, and much less in other ethnical groups and it is an auto-immune disease. It is also believed to be located on chromosome 6 in the HLA region. Some of the HLA genes passed into the human genome from Neanderthal and Denisovan gene pools might lead to boosted immunity and, in parallel, to autoimmune diseases, such as multiple sclerosis, psoriasis, rheumatism and eczema (Abi-Rached, 2011; Gibbons, 2011; Hüffmeier et al., 2010).
▪ Moreover, Factor V Leiden was introduced in the Northern European population 35 to 40 Ka. After trauma, the formation of a thrombus is essential to stem bleeding, but any too little clotting results in bleeding disorders such as haemophilia; it could be deadly as well as excessive clotting, which produces blood clots blocking the lungs. The most common inherited mutation that predisposes to thrombosis is the factor V Leiden mutation, a common mutation, with a prevalence of 2% in Caucasian populations (Bauduer and Lacombe, 2005).
▪ Finally, Freidreich Ataxia (FA) developed on a common haplotype only found in Europe, North Africa and the Middle East. It has been dated to 10 to 25 Ka. FA, like hemochromatosis, is associated with iron transport. The primary cause of FA seems to be oxidative damage by free radicals. Either there was a protective allele in Neanderthals against free radicals, or some mutation in the mitochondria protected them. In the first case, this allele might still be in the modern human gene pool, in the second, it was lost in the hybridization process (Colombo and Carobene, 2004).
▪ In fact, extensive research during the two last decades has confirmed an Ayuvedic truth dated back to 3,000 BCE. Chronic oxidative stress leads to chronic inflammation (due to microbial and viral infections, exposure to allergens, radiation and toxic chemicals, autoimmune and chronic diseases, obesity, consumption of alcohol, tobacco use, and a high-calorie diet), a triggering mechanism for diabetes, cancer, cardiovascular, pulmonary and neurological diseases (Reuter et al., 2010).
On the other hand, the natural glucocorticoids (steroid hormones with powerful anti-inflammatory effects produced by the human body in the cortex of the adrenal gland) are involved in glucose (sugar) metabolism - especially during times when no food is being taken in by the body - by: (1) stimulating glucose production in cells, particularly in the liver, (2) stimulating fat breakdown in adipose (fat) tissue and (3) inhibiting glucose and fat storage in cells. They have both metabolic and anti-inflammatory effects. And there is a strong relationship between HD and inflammation processes. Research has shown that glucocorticoids could play essential roles in delaying or inhibiting the progression of diseases such as HD (Huntington's disease), SBMA (Spinal and bulbar muscular atrophy expressed in males), and possibly other polyglutamine diseases as well.
Genetic studies performed in multiple modern human populations identified also disease risk alleles that are common in one population but rare in others. For example, a gene variant that seems to increase the risk of diabetes in Latin Americans and East Asians appears to have been inherited from Neanderthals.
In parallel, the SLC16A11 sequence is found in a newly sequenced Neanderthal genome from Denisova Cave in Siberia (The SIGMA Type 2 Diabetes Consortium, 2013). Moreover, genes involved in lipid catabolism detected in remains from Neanderthal sites is three times more frequent in contemporary Europeans than Asian and African populations (Khrameeva, et al., 2014). In addition, another recent study has shown that a hypoxia pathway gene, EPAS1 (which regulates the body’s production of haemoglobin), was previously identified as having the most extreme signature of positive selection in Tibetans, and a very low frequency among Han Chinese, explained by introgression of DNA from Denisovan or Denisovan-related individuals into modern humans (Huerta-Sánchez, et al., 2014).
▪ Phenylketonuria (PKU) is an autosomal recessive metabolic genetic disorder that can cause problems with brain development, leading to progressive mental retardation, brain damage, and seizures. Today, it is treated with a low-phenylalanine diet, which alone may not be enough to prevent the negative effects of phenylalanine levels; there is currently no cure for this disease. It is heterogeneous; more than 300 different mutations in the phenylalanine hydroxylase (PAH) gene have been identified. The heterozygote advantage seems to function as follows: The mild, wet climate of the Eucratic Zone tends to encourage the growth of moulds. In areas suffering repeatedly of famines, economic hardship and periods of poor nutrition over many centuries, food prepared from mouldy grain, which would otherwise be discarded, tends to be eaten; such food is likely to contain the fungal toxin ochratoxin A, which causes pregnant women to miscarry. But, if they are heterozygous for PKU, the higher concentration of phenylalanine in their blood would tend to protect the foetus (Woolf, et al., 1975; Woolf, 1996). It is noteworthy that PAH is closely related to two other homologous enzymes (tryptophan hydroxylase, which controls the levels of serotonin in the brain and the gastrointestinal tract, and tyrosine hydroxylase, which controls the levels of dopamine, epinephrine, and norepinephrine in the brain and the adrenal medulla). Recent research suggests that PKU may resemble amyloid diseases, such as Alzheimer's disease and Parkinson's disease, due to the formation of toxic amyloid-like assemblies of phenylalanine (Adler-Abramovich et al., 2012).
▪ Huntington's Disease (HD), which causes progressive damage to the nervous system (uncontrollable movements, dementia, and psychiatric disturbances), is a neurodegenerative genetic disorder of Western European origin. Having a prevalence of 15 per 100,000 in some Western European populations, it is rare in Asians and Africans. All humans have two copies of the Huntingtin gene (HTT), which codes for the protein Huntingtin (Htt). The mutation is genetically dominant and almost fully penetrant, meaning that mutation of either of a person's HTT genes causes the disease.
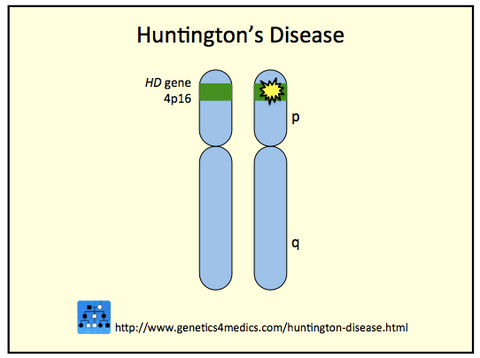
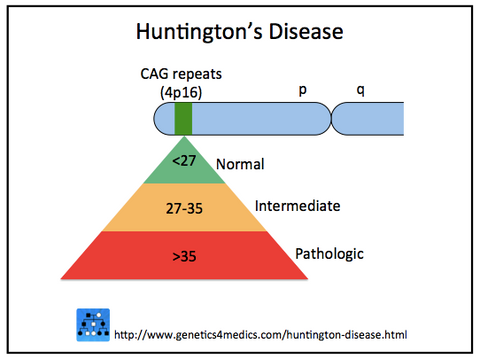
leading to increasing disease severity and/or decreasing age of onset.
Image credit: Genetics 4 Medics. http://genetics4medics.com/huntington-disease.html
The HD allele seems to protect against cancer (Sørensen, et al., 1999) and infectious disease (very much needed, because Neanderthals also carried the Cystic Fibrosis alleles). It might even play a key role in their abilities to hibernate. EPA and DHA protect brain areas affected by HD in hibernating mammals, and play a role in relieving ADHD symptoms. In detail, the omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are conditionally essential anti-inflammatory nutrients that enhance the quality of life, lower the risk of premature death, and generate neuroprotective metabolites by functioning exclusively via cell membranes, in which they are anchored by phospholipid molecules. DHA is proven essential to pre- and postnatal brain development, whereas EPA seems more influential on behaviour and mood (Kidd, 2007).
▪ Cystic fibrosis (CFTR) is a very diverse genetic disease, estimated to have been introduced 50 Ka in Europe, bearing also many similar properties to Coeliac, and might in fact be related to gluten-intolerance. The purpose of CF probably was to protect against infection and parasites (e.g. against cholera or typhoid fever). CF is associated with high levels of salt in sweat, and this probably formed some sort of protection, if left on the skin. This genetic mutation of cystic fibrosis in humans seems to offer a selective advantage against Vibrio cholerae infections, being a similar mechanism to the heterozygous carriers of Thalassaemia against malaria (Bertrandpetit and Calafell, 1996). Recently, researchers have plugged data from historical death rates for cholera, typhoid and tuberculosis (TB) into a complex demographic model (Poolman and Galvani, 2007).
▪ Studies confirms a link between low IgA levels, otitis media and autoimmune diseases, like Coeliac Disease (CD), also known as gluten intolerance (Simoons, 1981). Many autistics have lower than normal IgA levels.
The disease mostly affects people of European descent, and occurs more rarely in African and Asian populations. Neanderthals lacked nearly the entire gene variants linked to Coeliac disease, suggesting that the disease developed more recently, as humans evolved genetic resistance to epidemic diseases.
▪ According to the latest research findings, the modern human genome, compared with the Neanderthal and Denisovan genomes (reflecting low starch diets), on average possesses three times more copies of the gene for salivary amylase (AMY1 = an enzyme in saliva that helps break down starch). It seems that these modern human gene duplications occurred in the last 600 Ka, after the split between Neanderthals and Denisovans. AMY is a salivary endo-enzyme responsible for hydrolysis of α-1,4 glycosidic linkages in starch to produce maltose, maltriose, and other oligosaccharides, and it is the most abundant protein in human saliva, but is also highly variable. Something like 70% of the calories in human agricultural populations have been taken from the consumption of starch. Scientists hypothesized that dietary preference for high starch foods prevailed because individuals with more copies of AMY1 may be protected against death from diarrheal and intestinal disease (Perry, et al., 2007; Mandel, et al., 2010; Xu and Sin, 2012; Perry, et al., 2015).
▪ Studies have shown that human blood group O, and a negative Rhesus factor, may have been in the majority among Neanderthals. In fact, at least two of the extinct, ancient humans had type O blood, making them ‘universal donors’. Modern research indicates that this mutation took place about 1 Ma, probably in the hominid who was a common ancestor of humans and Neanderthals, as Natural Selection may sometimes favour a specific blood type. Type O seems to protect against severe malaria, tuberculosis, or syphilis, for example, but makes people more susceptible to bacteria that cause severe cholera and stomach ulcers, to Hypothyroidism and higher risk of venous thromboembolism, as well as to lower egg count and poorer egg quality in women, affecting the chances of conceiving, and to male infertility rates (Mourant, et al., 1978; Borιn, et al., 1993; Swerdlow, et al., 1994; Fry, et al., 2007; Lalueza-Fox et al., 2008; Khan, et al., 2010; The 66th American Society for Reproductive Medicine – ASRM Annual Meeting 2010: study from Yale University and the Albert Einstein College of Medicine).
▪ Very few extinction (or near-extinction) events seem to have only one cause. A bacterial pandemic triggered by the weakening of immune systems due to other factors, such as starvation or airborne/waterborne pollution following the colossal Toba eruption, is, also, a very logical mechanism proposed by researchers for the Neanderthals’ extinction. New genetic evidence suggests that those bacteria (e.g. Group B Streptococcus and Escherichia coli K1) were exploiting two genes of the human immune system, called Siglec-13 and Siglec-17, which are by now inactivated. This process was long, dating between 440 and 270 Ka. But some people may have had working versions of Siglec-13 as recently as 46 Ka. During that period, our ancestors were decimated by disease (Wang, et al., 2012).
▪ Over 50% of people with schizophrenia are rhesus negative, and there are indications of similar frequencies in Autism and Asperger (Hollister, et al., 1996).
One other major hypothesis seems also to be confirmed. The evolutionary origin of Bipolar Disorder (EOBD) shows the involvement of the circadian gene network in its pathophysiology; it is correlated with a cold-adapted build, and its moods vary according to light and season. Considering that selective pressures during the Pleistocene would have been greatest for women of reproductive age, they are expected to manifest winter depression more than males or younger females, which is the case. Thus, Neanderthals are considered as the ancestral source for bipolar vulnerability genes (susceptibility alleles). Researchers go beyond seasonal affective disorder, too, suggesting a probable annual hibernation period for Neanderthals who lived in the extreme cold conditions of higher latitudes (Kretchmer, 1970; Rosenthal et al., 1984 & 1987; Wehr and Rosenthal, 1989; Ciarleglio, et al., 2011; Sherman, 2012).
▪ Researchers looked also for relatively new mutations that might counteract a tendency towards dementia. They found it first in the gene CD33, which encodes a receptor that keeps inflammation and the immune response in line. The “C” allele is associated with late-onset dementia but the “A” allele protects cognition by enabling microglia (a type of brain cell) to sop up excess amyloid beta, one of the sticky proteins that accumulates in Alzheimer’s. Chimps, bonobos, gorillas, Neanderthals, and Denisovans all have the “C” allele, with some lucky modern humans the only primates examined to have the protective “A” allele. The A variant is thought to have emerged about 550,000 to 765,000 years ago.
In addition, researchers identified 10 other genes that have variants protecting against the vascular form of dementia (APOE, AGT, SCG2, CAPN10, TCF7L2, EBF1, COX-2, CYP3A5, PPARG, and PON1). Instead of drowning brain cells in amyloid beta and tau proteins, vascular dementia blocks blood flow. These 10 genes protect against hypertension, type 2 diabetes, and cardiovascular disease. These “derived protective alleles” are found in all modern African populations, indicating that they are at least 100,000 years old (Schwarz et al., 2016).
▪ Recently, researchers have also identified the ARHGAP11B gene as a hominin-specific gene (found in Neanderthals, Denisovans and modern humans), that drives the proliferation of the neural progenitor cells that build the brain’s neocortex. Sensory perception, motor commands, conscious thought, and language are involved in this brain area (Florio, et al., 2015).
▪ In the contrary, the dopamine receptor subtype DRD4 7R gene is associated with risk-taking, sensation-seeking, spatial orienting of attention and novelty-seeking, and is correlated with openness to new experiences, intolerance to monotony, and exploratory behaviour (temperament dimension of novelty seeking - NS). According to the human genome, the DRD4 7R gene suddenly showed up about 37 Ka, it spread due to natural selection and is unlikely to be of modern human origin. In modern populations, 10% have the activated DRD4 7R gene, 20% are just carriers and ca 70% don't have it at all (Swanson, et al., 2000; Ding, et al., 2002; Wang, et al., 2004; Lundwall, et al., 2012). This gene increases also peripheral vision.
▪ Moreover, in overall size, the posterior portion of the Neanderthal brain, for example the occipital and superior parietal lobes, were slightly larger in length and breadth than the modern human brain on average. Scientists consider this as a reflection of the environment in which they dwelled, and the neural capacities their life style required. Additonally, the Neanderthals’ temporal lobe was well developed, bigger and little different from that of modern humans, as physical indices along with examination of their skulls and endocasts have shown. The limbic system (amygdale, hippocampus, temporal lobe) controls sexual stimulation, memories (of body and mind), feelings of love and affection, and the ability to form long-term attachments, as well as dream sleep (visual, emotional, and hallucinatory aspects), violence, murder, religious and spiritual experiences. Archaeological evidence supports the anthropological framework. Anthropological evidence has demonstrated that the frontal lobes significantly expanded in length and height during the Middle to Upper Palaeolithic transition, an estimated increase in size by almost a third in the transition from archaic humans to Cro-Magnon. Generally speaking, frontal lobes are considered as the senior executive of the brain, responsible for initiative, goal formation, long term planning, the generation of multiple alternatives, the consideration of multiple consequences, and free will; they coordinate and regulate intellectual, creative, artistic, symbolic, and cognitive processes (Joseph, 2011).
▪ In addition, the latest research conducted claims that the absolute and proportional frontal region size, which increased rapidly in humans, was tightly correlated with corresponding size increases in other areas and whole brain size, and with decreases in frontal neuron densities. Thus, the search for the neural basis of human cognitive uniqueness should focus more on distributed neural networks, than on the frontal lobes in isolation (Barton and Venditti, 2013). Nevertheless, evidence shows that Homo symbolicus included both Neanderthals and Sapiens (for a short integrated perspective of human emotions see Tarlow, 2000; Henshilwood and d’ Errico, 2011; Panksepp and Biven, 2012). Even more, a zigzag engraving on a shell - from a freshwater mussel species - found in Trinil, Java (Indonesia) in the 1890s by the Dutch palaeontologist Eugène Dubois, is the oldest abstract marking ever found, dated to 500 Ka, and its likely creator seems to be the common human ancestor Homo erectus (Joordans, et al., 2015). The shell is included in the Dubois collection (Naturalis Museum, Leiden, The Netherlands). The astonishing finds and constructions at Bruniquel Cave (southwestern France) dated to ca 176.5 Ka (±2.1 Ka) proved that Neanderthals mastered the underground environment in a unique and “modern” way at a 336 m distance from the entrance (Jaubert et al., 2016).

Bruniquel cave. Image credit: Michel Soulier – SSAC / Nature Jaubert et al
▪ Ancient origins more than 100 Ka of a set of Y-chromosome mutations not found in Africa is indicative of a Neanderthal and/or Asian Homo erectus contribution to our genome (Underhill, et al., 1997).
Neanderthals have distinguished into at least four subgroups, as they were not a homogenous group. Paleoanthropological studies based on morphological skeletal evidence have offered some support for the existence of four different sub-groups, one in Western Europe, one in Southern Europe, one in the Levant, and another in Western Asia (Fabre, et al., 2009). Although many scientists question the interbreeding theory (Ander and Manica, 2012), genetic research has shown two periods of interbreeding between modern humans and Neanderthals; one period of interbreeding occurring around 60 Ka in the Eastern Mediterranean, the other around 45 Ka in Eastern Asia (Krause, et al., 2010). The most recent and detailed genetic analysis of the existing data indicates that the divergence between modern humans and Neanderthals took place between 270 and 440 Ka, a common ancestor of both having lived within the last 500 to 800 Ka (Green et al., 2008).
Furthermore, their later interbreeding is reflected in the genome differences in the degree in which different geographically dispersed present-day population groups show Neanderthal ancestry. According to a first approach by the scientists involved in ‘1000 Genomes Project’, more Neanderthal indicators have been found in Northern China populations in relation to those of Southern China, and in the Southern populations of Europe more than in the northern, with the Tuscans having the highest level of Neanderthal similarity (The 1000 Genomes Project Consortium, 2010; The Malapa Soft Tissue Project, by John Hawks, online at: http://johnhawks.net/malapa).
Research on singularities in the Neanderthal genome has also shown 78 genes that have been located with changes, but because of the low genome coverage, there could be up to three times more. Some genes on the list are easy to interpret, for example, the changes in five olfactory receptors, which are probably inactivated nowadays because they are no longer important to our survival (Green, et al., 2010). Some different genes between Neanderthals and Homo sapiens sapiens are: RPTN (gene that encodes repetin, an extracellular epidermal matrix protein, involved in the sweat glands, the root hairs and tongue papillae), SPAG17 (an important antigen for the structural integrity of the central apparatus of the sperm axoneme, it participates in male fertility), TTF1 (regulates ribosomal gene transcription), DCHS-1 (encodes for a protein involved in wound healing), CAN15 (encodes a protein of unknown function) and PCD16 (fibroblast cadherin, encodes a cell-cell adhesion protein, possibly involved in wound healing).
Evidence provided by a jawbone found in 2002 inside the cave system of Peștera cu Oase in Southwestern Romania revealed that the Neanderthal signature of the Oase individual's genome ranges between 6% and 9%, making it an unprecedented amount because present-day Neanderthal derived European genome range between 2% and 4%. That ancient man seemed to have a Neanderthal ancestor just four to six generations back, but he was also more closely related to modern East Asians and Native Americans than to today's Europeans (Fu, et al., 2015).
▪ Finally, skin colour, hair colour, freckles and eye colour are related to three mutations in the melanocortin-1 receptor found on chromosome 16 - MC1R (R151C, R160W and D294H), the origin of which have been traced back to 50 to 100 Ka, as a result of hybridization with Neanderthals. Recent research has demonstrated that people with red hair have different sensitivity to pain compared to people with other hair colours, and naturally occurring low vitamin K levels (Mogil, et al., 2005; Liem, et al., 2005; Lalueza-Fox, et al., 2007). In parallel, researchers found that the Neanderthal genome affects keratinocytes that protect the skin from environmental damage such as ultraviolet radiation and pathogens. Those variants influence skin biology in modern humans too, in particular, the risk of developing sun-induced skin lesions called keratosis, which are caused by abnormal keratinocytes (Simonti et al., 2016; see also: https://pharmaceuticalintelligence.com/2016/02/13/genomic-expression-carried-over-from-neanderthal-dna/; http://www.eva.mpg.de/neandertal/index.html).
The Eternal Embrace

NEW: The Eternal Embrace

Articles in Q-MAG.org
Cyprus salt lakes exonerate Peoples of the Sea from destroying Bronze Age civilizations
Toppling Rome's obelisks and aqueducts
Trevor Palmer's response to Gunnar Heinsohn
Jan Beaufort: Conspiracy or religious history?
Gunnar Heinsohn's answer to Trevor Palmer
G.H.: Vikings without towns, ports and sails...
Trevor Palmer challenges Gunnar Heinsohn
The 1st Millennium AD controversy
The exact dates of the deaths of Patroclus and Hector
Lybian rock-art erased by Jihadists
The tsunami that obliterated Doggerland
Miles below ground, live the creatures of the Deep
Navigation systems of migratory birds suffer from electromagnetic fields
G.H.: Charlemagne's correct place in history
The petroglyph sundial of Mount Bégo
Mount Bégo: an electrical mountain

